Sodium Hydroxide Molar Mass
The density of 50 ww Sodium hydroxide solution is 1515 gml at 25C which means that the weight of the 1 ml of Sodium hydroxide solution is 1515 g at 25C. Sodium hydroxide CAS 1310-73-2 pellets EMPLURA - Find MSDS or SDS a COA data sheets and more information.

Quiz Worksheet Molar Mass Study Com
399971 gmol Appearance White hard when pure opaque crystals Odor.

. Solubility at 20 0 C. NaOH HOOC-C6H4-COOK Æ NaOOC-C6H4-COOK H2O By measuring the volume of the 02M NaOH solution dispensed from the buret that is necessary. Molarity refers to the number of moles of the solute present in 1 liter of solution.
596 K Boiling point. Properties of Sodium Hydroxide NaOH In order to describe its uses it is necessary to describe the features that caustic soda is used in a variety of industries due to these chemical and physical features. Download Product Safety Card.
Sodium AcetateCH3COONa- Sodium acetate is the salt of acetic acid and sodium hydroxide. Reduced boiling point and freezing point. Exhaust from a chimney contains 10 mol of oxygen O 2 53 mol of nitrogen N 2 and 37 mol of carbon dioxide CO 2.
The molarity of a sodium hydroxide solution is 051 M. A 50 ww sodium hydroxide solution means that there is 50 g of NaOH per 100 g of solution. Thus the most common way to determine the concentration of any sodium hydroxide solution is by titration.
It is hygroscopic in nature and easily soluble in water. The value of the Vant Hoff factor is less than one. Determining the precise concentration of NaOH using a primary standard is called.
Molar mass is the mass of 1 mole of the solute. 106462 View Pricing Availability. Hydronium and hydroxide ions in pure water at 25 C.
Specific Gravity at 20 0 C. Calculate the molar concentration of the sodium hydroxide solution using your data for each run. The reaction between NaOH and KHP molar mass 20423 gmole is as follows.
The value of i is greater than one. 323 C 613 F. The density of the solution is 102 g cm 3.
Of sodium hydroxide present using the mass recorded since any sample of sodium hydroxide is likely to be a mixture of sodium hydroxide and water. Observed molar mass is greater than the predicted value. Molecular Weight Molar Mass.
Pure ideal gascalc435 at 0 C and 101325 kPa. Calculate the average. It is widely used across a number of industrial sectors.
It is a constant property of each substance for example the molar mass of water is approximately equal to 18 gmol. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. 40 gmol Chemical Formula.
It is expressed in grams per mole. The values of the colligative properties are lower than expected. The molar mass of sodium hydroxide is 40 g mol 1.
Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed. The observed value of molar mass is lesser than the normal value. The molar mass of oxygen nitrogen and.

Sodium Hydroxide Naoh Molecular Weight Calculation Laboratory Notes

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube
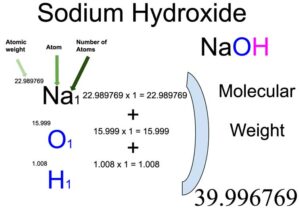
Sodium Hydroxide Naoh Molecular Weight Calculation Laboratory Notes
No comments for "Sodium Hydroxide Molar Mass"
Post a Comment